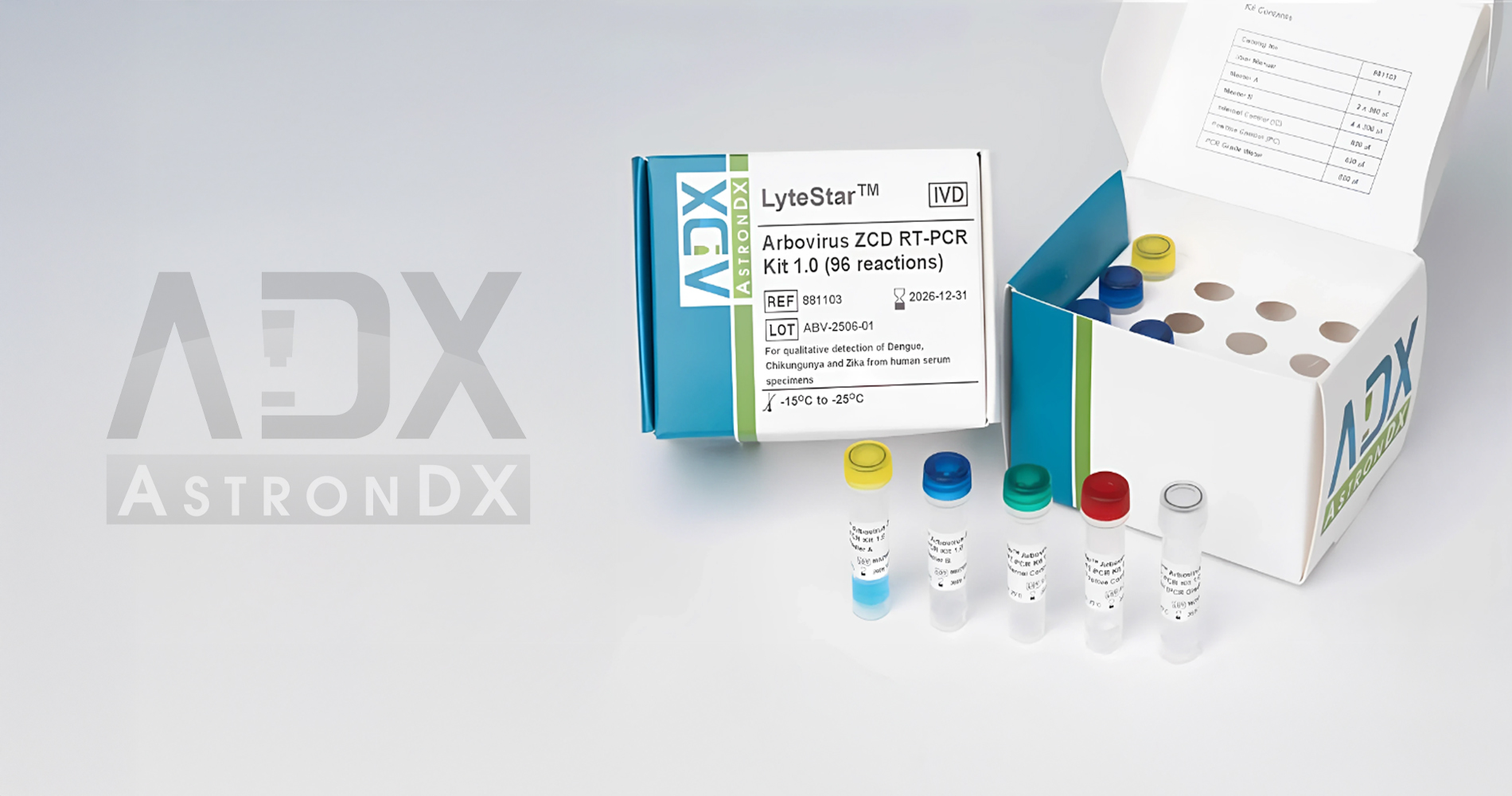
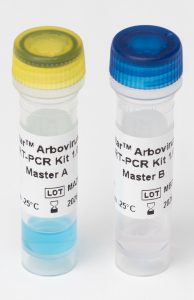
PCR Master A and Master B contain all reagents required for target amplification
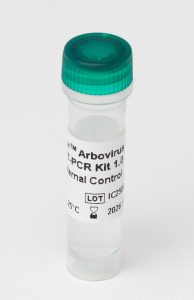
Internal Control (IC) monitors PCR inhibition and extraction efficiency
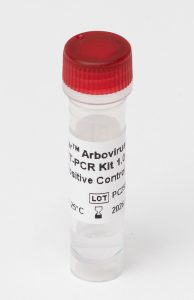
Positive Control (PC) monitors correct setup and ability to detect target
Non-Template Control (NTC) monitors contamination and excludes false positives
At AstronDX we fully embrace the challenges intrinsic to the creation of products with unsurpassed quality and performance, offering highest value for the specific needs of the communities and customers we serve. Our LyteStar™ detection kits are designed, developed, validated and manufactured in compliance with ISO 13485 and meet the regulatory requirements in our target markets.
Our dictum PER ASPERA AD ASTRON is not only an affirmation of our beliefs, values and aspirations and holding us accountable to our high standards but also serves as an invitation to our partners and customers to let themselves be guided by their own set of challenges to adopt an AstronDX product as a reliable, convenient and effective solution to their needs.
SARS-CoV-2, Influenza A, Influenza B, RSV
Pan-Enterovirus, EV71, Coxsackievirus A6 & A16

Any molecular diagnostic test can only deliver high quality results as long as the upstream sample processing and nucleic acid extraction provides starting material in the form of DNA or RNA in sufficient quality. The efficiency of nucleic acid extraction and the quality and purity of the extracted DNA or RNA has a fundamental impact on the PCR reaction.
With this well-understood fact at heart, we complement our range of LyteStar detection kits with a concise range of SpinStar extraction kits that offer a solution for any application.