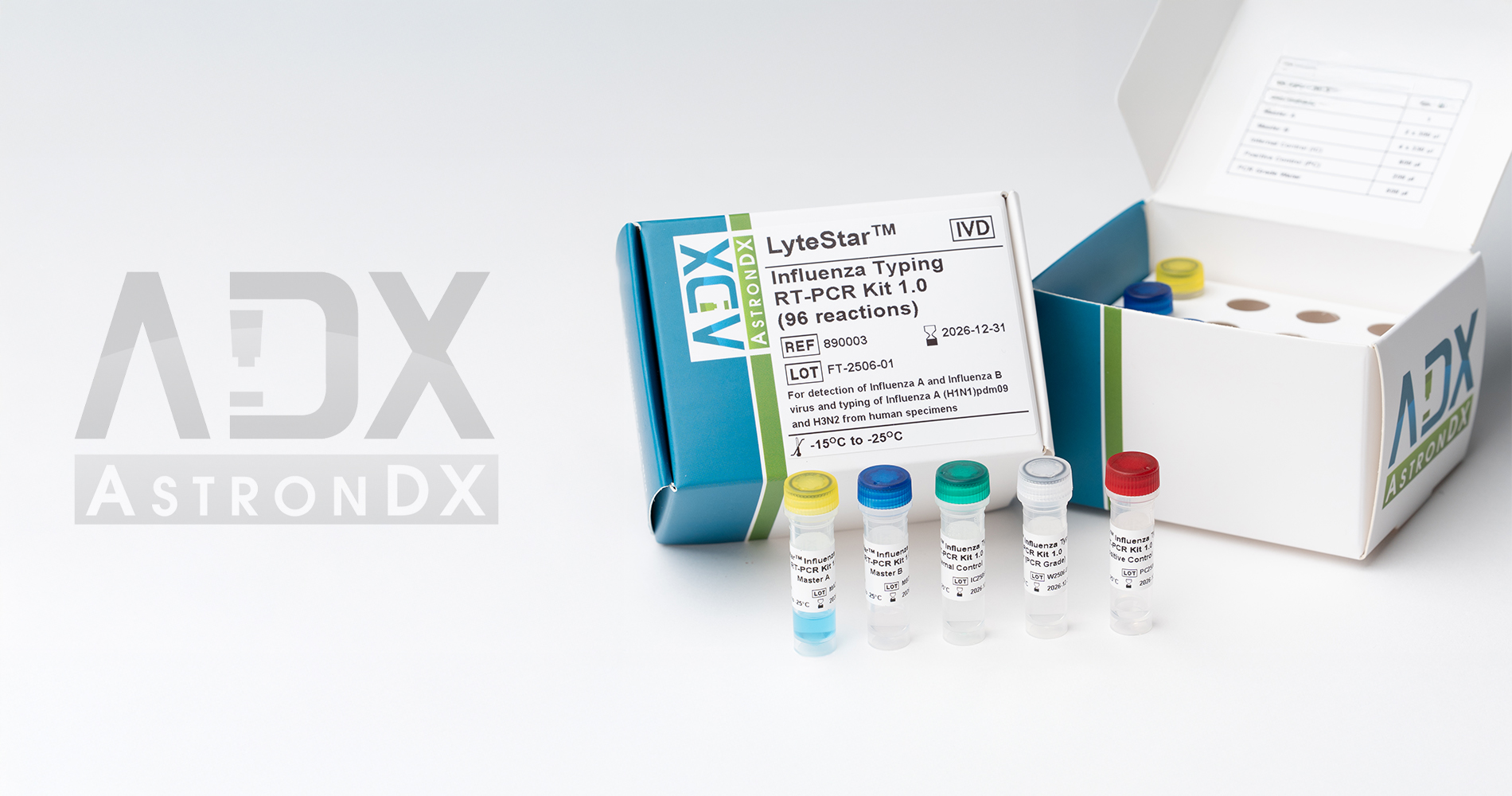
The LyteStar™ Influenza Typing RT-PCR Kit 1.0 is intended for the specific detection of Influenza A/B RNA in human respiratory specimens. The LyteStar™ Influenza Typing RT-PCR Kit 1.0 is a four-target assay comprising a screening assay targeting the Influenza A matrix (M) and Influenza B hemagglutinin (HA) genes. The assay further differentiates the Influenza A into A(H1N1)pdm09 and H3N2, via the HA gene.
|
Multiplex kit for Detection and Differentiation:
|
Highest Analytical Sensitivity
|
|---|---|
|
Influenza A specific; M-gene
|
0.28 cp/μl
|
|
Influenza B specific; HA-gene
|
0.41 cp/μl
|
|
Influenza A(H1N1)pdm09 specific; HA-gene
|
0.96 cp/μl
|
|
Influenza A/H3N2 specific; HA-gene
|
1.61 cp/μl
|
|
Internal Control (IC); heterologous RNA
|