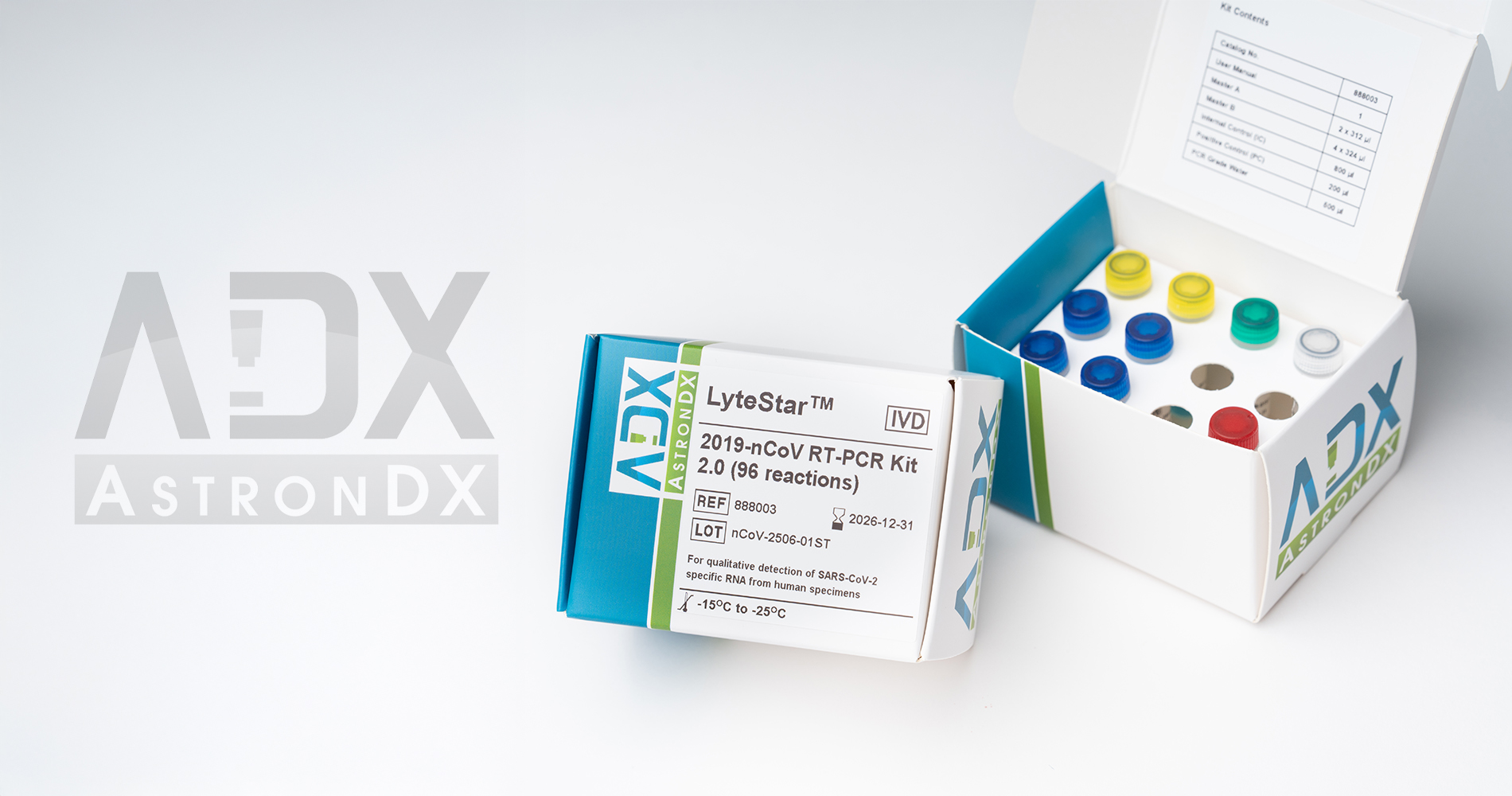
The LyteStar™ 2019-nCoV RT-PCR Kit 2.0 is intended for the specific detection of SARS-CoV-2 RNA in human respiratory specimens (bronchoalveolar lavage, tracheal aspirate, sputum, nasopharyngeal and oropharyngeal swabs placed in VTM, nasopharyngeal wash/aspirate, and nasal wash/aspirate). The LyteStar™ 2019-nCoV RT-PCR Kit 2.0 is a dual target assay comprising a screening assay targeting the E gene and a confirmation assay targeting the RdRP gene.
|
Multiplex kit for Detection and Differentiation:
|
Highest Analytical Sensitivity
|
|---|---|
|
SARS-CoV related CoV; E-gene
|
2.14 cp/μl
|
|
SARS-CoV-2 specific; RdRP-gene
|
1.68 cp/μl
|
|
Internal Control (IC); heterologous RNA
|