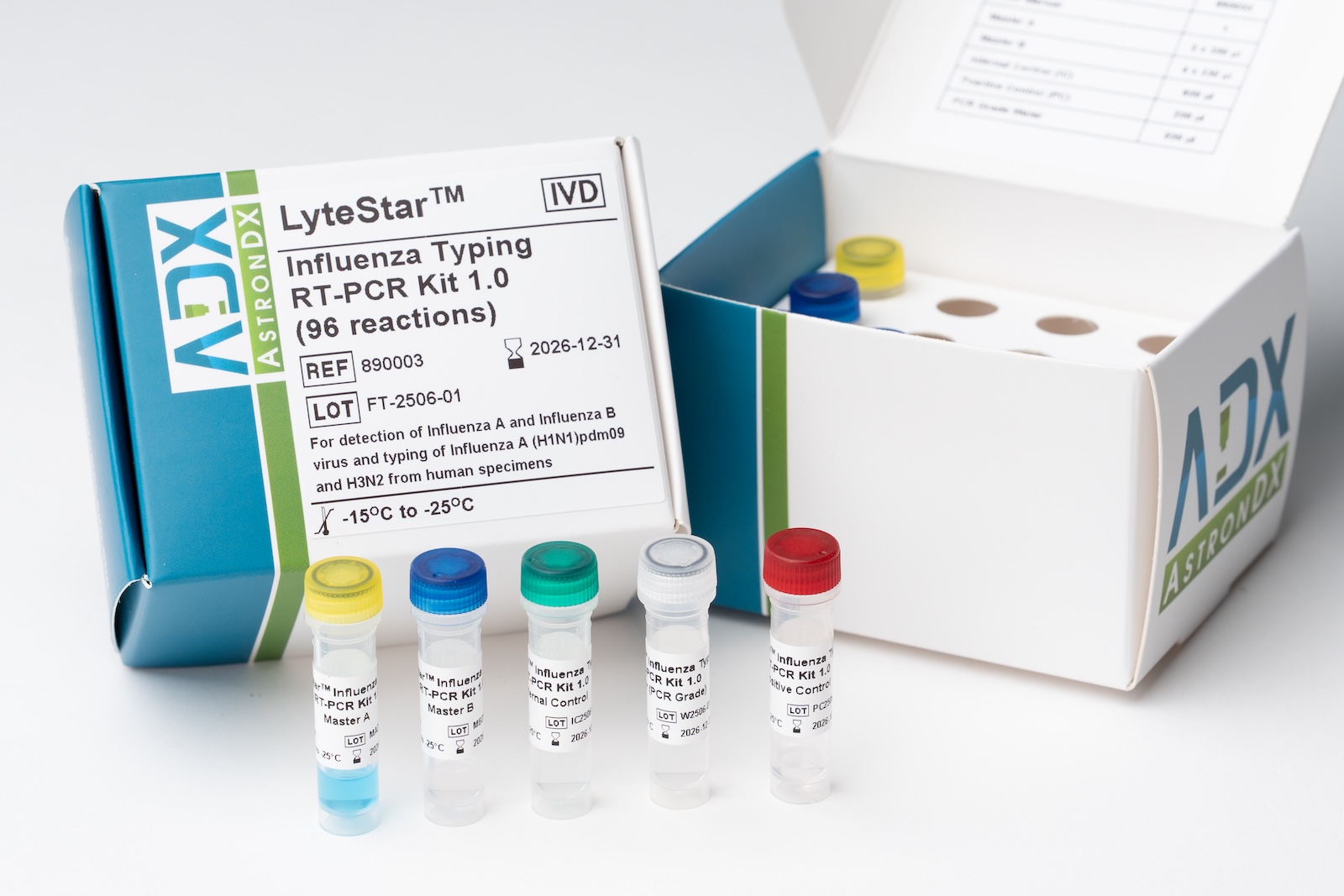
04/2025 – ADT Molecular has been awarded by the Johor Bahru regional Public Health Laboratory (Makmal Kesihatan Awam Johor Bahru, MKAJB) to supply the LyteStar™ Influenza Typing RT-PCR kit for Influenza surveillance screening in the southern part of the Malaysian peninsular. The award follows the regulatory approval of the product by the Malaysian Medical Device Authority (MDA) in 2023 and successful multi-center evaluations delivering consistent high quality performance and reliable, accurate results. The award is another important milestone in our commitment to deepen our contribution and provide value to the patients and communities we serve, as well as delivering growth and perspective as a BioNexus company contributing to the National Biotechnology Policy 2.0, a key government initiative fostering the transition of Malaysia towards a knowledge-based society.
The LyteStar™ Influenza Typing RT-PCR Kit 1.0 is an in vitro diagnostic test system, based on real-time PCR technology, for the qualitative detection and differentiation of Influenza A, Influenza B, Influenza A(H1N1), and Influenza A H3N2 specific RNA. The LyteStar™ Influenza Typing RT-PCR Kit 1.0 consists of four assays, one targeting the M gene for identification of Influenza A, the second targeting the HA gene that specifically detects Influenza B and two more assays targeting the Influenza A HA gene to differentiate between the Influenza A(H1N1)pdm09 and Influenza A/H3N2. The LyteStar™ Influenza Typing RT-PCR Kit 1.0 includes a heterologous amplification system (Internal Control) to identify possible RT-PCR inhibition and to confirm the integrity of the reagents of the kit.