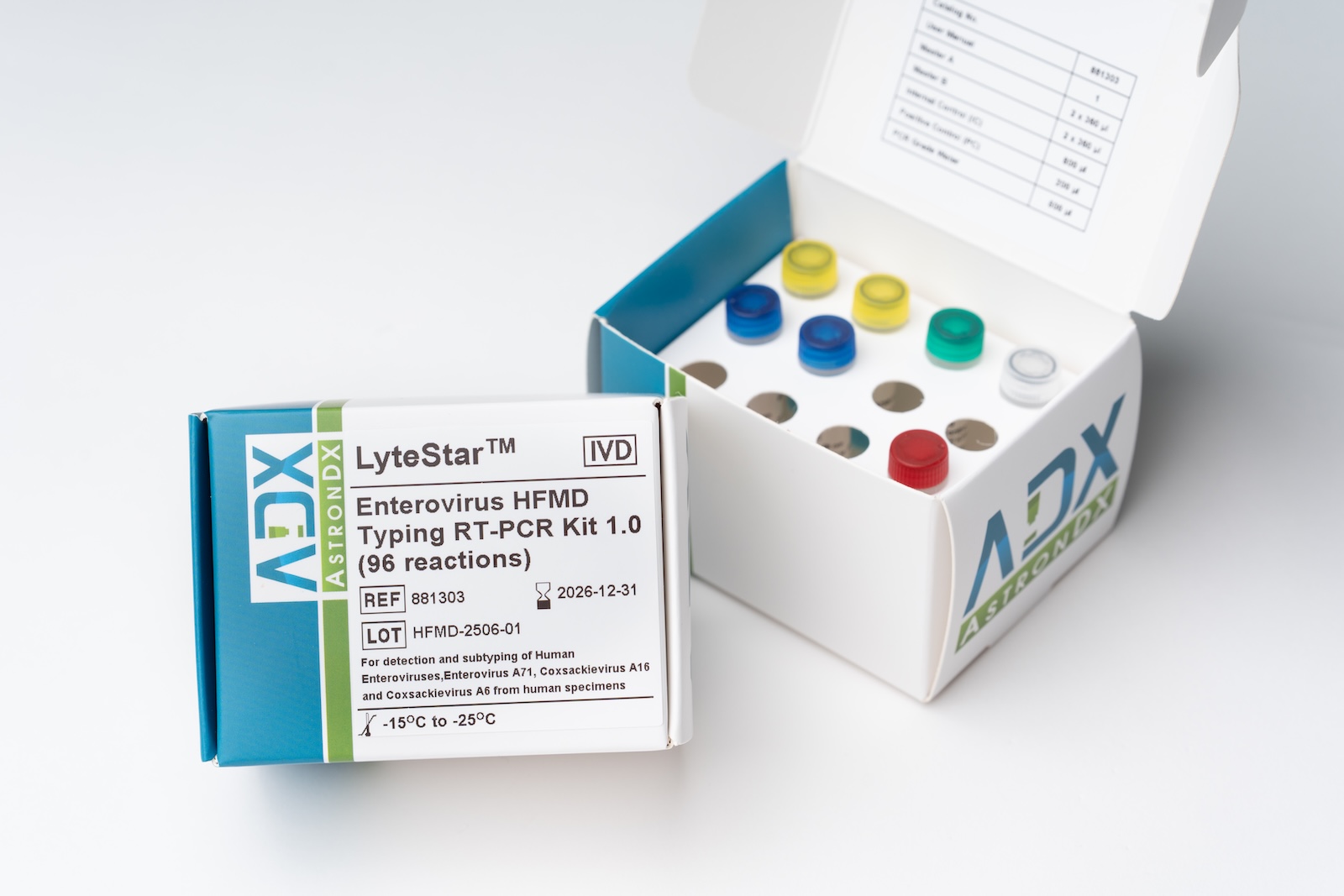
06/2025 – ADT Molecular has been awarded a tender by the Malaysian National Public Health Laboratories (Makmal Kesihatan Awam Kebangsaan) for the supply of our LyteStar™ Enterovirus HFMD RT-PCR kit. The kit will be deployed across the national surveillance network to support early detection and containment of Hand, Foot, and Mouth Disease (HFMD) outbreaks.
The award follows the regulatory approval by the Malaysian Medical Device Authority (MDA) in January 2025 and marks another significant milestone in our mission to deliver innovative molecular diagnostics to the Malaysian healthcare system. AstronDX remains deeply committed to advancing Malaysia’s National Biotechnology Policy 2.0, by delivering impactful solutions that strengthen public health and biosecurity.
The LyteStar™ Enterovirus HFMD Typing RT-PCR Kit 1.0 is an in vitro diagnostic test system, based on real-time PCR technology, for the qualitative detection of Human Enteroviruses (including Enterovirus A to D, Coxsackievirus A and B, Poliovirus 1, 2 and 3, and Echovirus; excluding Human Rhinoviruses) and subtyping of Enterovirus A71 (EV71), Coxsackievirus A16 (CVA16) and Coxsackievirus A6 (CVA6). The LyteStar™ Enterovirus HFMD Typing RT-PCR Kit 1.0 consists of a single tube assay with four targets, namely pan-Enterovirus (panEV), EV71, CVA16 and CVA6. The LyteStar™ Enterovirus HFMD Typing RT-PCR Kit 1.0 includes a heterologous amplification system (Internal Control) to identify possible RT-PCR inhibition and to confirm the integrity of the reagents of the kit.